Understanding the Vacuum Cycling Nucleation Process
VCN can offer enhanced surface treatment performance in both aqueous and solvent applications.
#basics
Many current industrial surface metal or composite material treatment operations, such as cleaning, passivation and etching, are limited by the mass transfer process of chemicals from bulk fluid to the part’s surface.
At the surface of parts there exists a distinct layer of fluid called the mass transfer boundary layer, where chemical concentrations and velocities are significantly lower than in the bulk solution away from the surface. In order to enhance chemical transferability through the mass transfer boundary layer, bulk fluid agitation (to decrease the boundary layer as much as possible) and/or increased chemical concentrations are needed.
Featured Content
But serious limitations still exist because of the boundary layer, where most of the resistance to mass transfer takes place; the fluid velocity used to convectively transfer the treating chemicals to or away from the surface is dampened and decreases rapidly near and at the solid surface. It is within this boundary layer that the rate of mass transfer slows because of a dependence upon molecular transfer mechanisms as opposed to the more rapid eddy transfer mechanism encountered in the bulk fluid region.
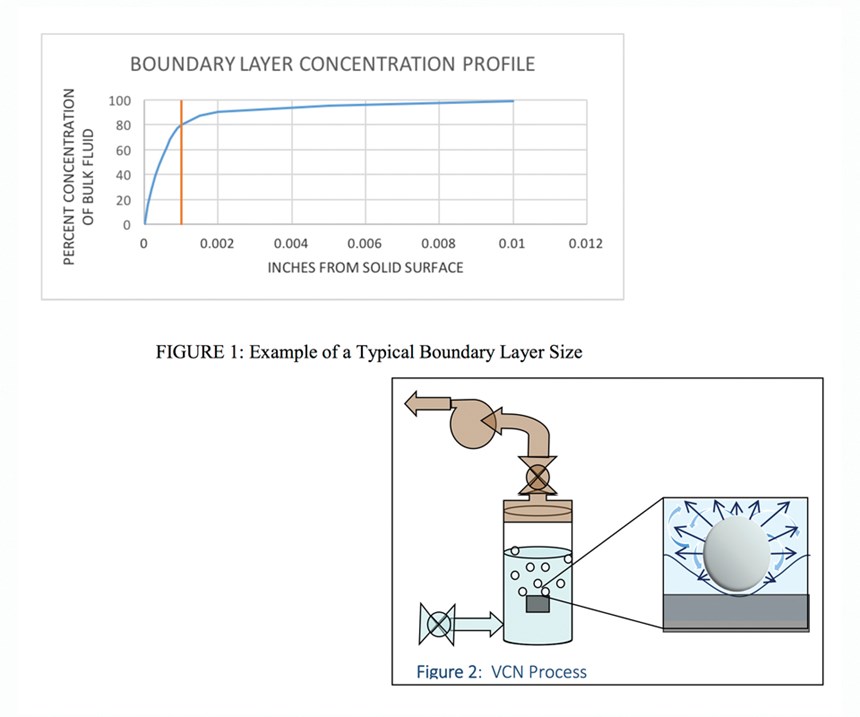
Since the mass transfer rate slows near a surface, a rapid change in the active ingredient chemical concentration occurs across this boundary layer. Figure 1 shows a typical concentration profile developed for a chemical reacting at the surface, thus showing a zero concentration at the surface.
Although the boundary layer in this example is only 1 mil, 80 percent of the change in concentration occurs across the 1-mil thick boundary layer. Increasing bulk concentration linearly increases the concentration within the boundary layer. Decreasing the boundary layer thickness shortens the length of the resistance path to the surface.
Reducing Total Pressure
The vacuum cycling nucleation (VCN) process is accomplished by reducing the total pressure in a controlled-environment chamber that contains (submerged in a solvent or an aqueous solution) the components to be cleaned. The reduced pressure results in the formation of vapor bubbles at the surface of parts, much like bubbles forming on the inside surface of a boiling pot of water. Typically, nucleation sites for bubble formation are found where imperfections, crevices or foreign particle material exist. Continuous evacuation of the chamber with a vacuum pump produces steady bubble formations on the solid surface; pulsing can also be applied to facilitate surface mass transfer.
As opposed to conventional methods used to reduce the fluid boundary layer near a surface, VCN bubbles nucleate at the solid surface and grow inside this boundary layer, subsequently disrupting the boundary layer. The mechanisms involved with these growing bubbles result in the movement of the boundary layer fluid (containing product chemicals or material removed from the surface) out into the bulk fluid; when the bubble grows large enough, it detaches from the surface and creates a flow of bulk fluid back into the surface region.
This type of forced convective mass transfer has been demonstrated to be up to an order of magnitude stronger than jetting or ultrasonic agitation. The low concentration of chemical in the bulk solution is brought to the nucleation site as a transiently “high” concentrated chemical that assists with surface mass transfer activity; as a result, any chemicals used in VCN are always maintained at a much lower concentration than used in typical surface preparation methods. An example of this phenomenon is shown in Figure 2, where a low concentration of chemical in the bulk solution exists. As the bubble is formed and released, the chemical is convectively brought to the surface, resulting in a higher concentration of chemical at the surface.
Intricate parts typically found in the medical device industry have cavities or recesses that involve more complicated boundary layer issues and require even more bulk fluid agitation and higher chemical concentrations to achieve the desired results. Ultrasonics are used to penetrate small recess areas. The problem is that ultrasonics can only act on recesses near the surface, and internal surfaces never see sonic bubbles. The diffusion process into deep recesses is extremely slow, and complete cleaning or surface treatment is rarely accomplished. Unsatisfactory surface treatment, high product rejection rates, long processing times and increased liability risks can lead to expensive products.
Tight areas are ideal nucleation points to grow VCN vapor bubbles. The high surface area to fluid volume ratio and absence of natural convection to cool the nucleation area help grow effective bubbles. The vapor bubbles force fluid from the part’s internal structure, and when pressure is applied, new bulk fluid enters the part. Cycling of vacuum and pressure removes contaminant or delivers fresh chemical for treating an internal surface.
Cleaning Steel Medical Tubing
VCN is currently being used to aqueously clean manufacturing fluids and debris from the internal surface of small lumen stainless steel medical tubing (hypodermic needles, for example). This high volume unit has two cleaning chambers supported by one cleaning supply tank, one rinse tank and one vacuum pump. The process cleans, rinses and vacuum dries nested bunches of medical tubing.
Another field unit is using VCN to clean sensitive electronic components contained within medical implants. The electronic network has tight areas; ultrasonics would damage the small electrical connections. VCN grows vapor bubbles within the network that are much gentler relative to ultrasonic bubble implosion. Damage to the sensitive electronics has been eliminated with faster and better cleaning.
VCN has been in use for years to clean chromatography components of manufacturing polishing compound from areas as small as 6 mils using an aqueous solution. The VCN process has eliminated a high rejection rate of parts cleaned with ultrasonics.
Large areas can also be problematic when cavities or channels are deep or have large aspect ratios. Areas shielded with solid walls can dampen forced convection methods or ultrasonics and prevent any penetration of processing fluid chemistries. VCN can expand fluids to five times their volume, thus ejecting the bulk of fluid within these internal areas. The expansion of vapor produces the agitation needed to flush the parts’ internals, and the cycling process assures a rapid turnover of fresh bulk fluid to guarantee a complete and uniform process.
A unit that has been in operation for more than a year in the field is being used to remove filler from 3D printed parts. In a 3-percent aqueous solution, parts normally requiring days to remove filler were cleaned in 10 minutes to four hours, depending upon the complexity of the part.
Range of Applications
The VCN process works just as well in solvent systems as in aqueous systems. One solvent system in the field is presently cleaning machining oil from tightly wound copper and aluminum rolls of screen. The screen has a small mesh size of 3 to 4 mil wire wound into rolls three feet long and 8 to 10 inches in diameter. The solvent soaks into the mesh, and when the vacuum is pulled, the solvent vapor expands, forcing fluid through the mesh and out into the bulk fluid. Applying pressure fills the mesh with fresh solvent. A two-second cycle applied for four minutes cleans the mesh. The rolls are cleaned, then vacuum dried. Solvent is recycled using on-board vacuum distillation.
VCN is being used in a range of applications and is continuously expanding to more applications. Applications include small and large tubes, delicate electronics, 3D printed parts, critical clean instrumentation, porous materials, medical devices and more. Processing can include cleaning, surface preparation, etching, coating, anodizing, sterilizing, passivation and more. Combining VCN with ultrasonics covers most present day cleaning and surface preparation requirements.
When used in aqueous applications, the VCN process typically requires a lower concentration of cleaning solution. In solvent applications, VCN can be combined with a vacuum solvent management process to achieve the highest environmental and safety standards.
RELATED CONTENT
-
Understanding CNC Collet Chucks
Workholding for turning is usually fairly basic: The selection comes down to chucks or collets. This article looks at when to consider the collet chuck and what kind might be best for a given application.
-
5 Grinding Considerations for Improving Surface Finish
Improving surface finish can be done by making adjustments to one or more of these points: operational parameters, wheel dressing, grit size, coolant delivery and machine condition.
-
Important Machining Factors of Carbon Steels
Learn the factors that contribute to carbon steels and their machinability as well as the 1214 steels, 1215 steels and 1018 steels, other grades, and more.