How to Reduce Steps Required in Nonferrous Surface Finishing
Appears in Print as: 'Reduce Steps for Nonferrous Surface Finishing'
The finishing of parts made from various copper and aluminum alloys traditionally require many distinct and separate process steps. Hubbard-Hall’s Mike Valenti explains how both come with their own unique needs .
#basics #columns
The cleaning and surface preparation of nonferrous, soft metals can require multiple and complex steps. Manufacturers using these alloys continue to require more efficient and less complex processes.
The finishing of parts made from various copper and aluminum alloys traditionally require many distinct and separate process steps, and both come with their own unique needs. Below we discuss the specific challenges that come with finishing copper and aluminum.
Copper alloys that include 99.9% copper, berylium copper (97.75% Cu, Be 2.0%), yellow brass (65% Cu, 35% Zn), and cartridge brass (70% Cu, 30% Zn):
- Cleaners — most brass and copper processes currently utilize alkaline-based cleaners (pH over 7.0). While very effective at removing lubricants, oils and metal shavings, these do not offer any ability to remove surface tarnish or add brightness to the parts. Traditional acid-based cleaners are typically not effective at removing these soils.
- Deoxidation — acids are used to deoxidize brass alloys. Organic acid salts and/or strong mineral acids are normally used.
- Brightening — for parts requiring a high luster or more polished look, strong mineral acid bright dips or peroxide-based chemical polish is required.
- Final Seal — copper and its alloys are highly susceptible to tarnish so a seal or antitarnish is needed to preserve the finish.
- Waste Treatment — while not an actual step of the parts finishing process, the waste treatment process can be greatly affected by the chemistries used and copper waste generated.

Ka =the acid dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution.
Aluminum alloys that include 1,000, 3,000, 5,000 and 6,000 series:
- Cleaners — aluminum parts can have difficult lubes, oxides and finely divided aluminum that must be removed.
- Etching/Smut — heavy alkaline or mineral acid-based processes can lead to over etching or smut. Smut removal requires additional steps using nitric acid.
- Equipment and Waste Treatment — silicates used in typical aluminum cleaners can reduce etch and smut, but contribute to scale buildup in equipment and waste treatment problems.
Some of the most challenging soils to remove from aluminum are lubricants made from stearates. These stearate lubes can react with aluminum during high-energy processes such as impact extrusion (deep drawn), making them problematic. Stearate contamination can lead to discoloration during heat treat steps, and adhesion issues during coatings or printing processes.
While these process steps have been proven over time, they present the following challenges:
- Many steps result in higher probability of a problem down the line, such as contamination and maintaining clean rinses.
- Handling of more hazardous chemistries, including concentrated mineral acids (sulfuric and nitric acids), fumes, peroxide stability, powders and dust. Copper, zinc and lead contamination in cleaner tanks requires chelators to prevent redeposition on parts and reduce scale buildup. These problems require more frequent tank dumps and maintenance. Over etching of parts results in rejects.
- Chelators interfering in waste treatment operations. Offsite waste disposal costs.
- Phosphates from cleaners.
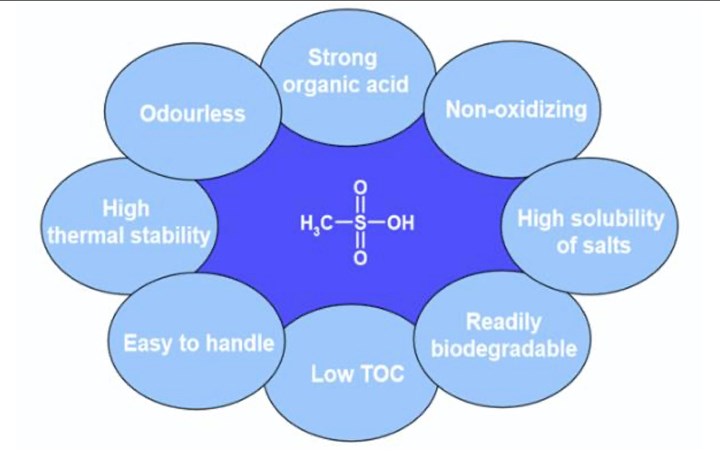
The properties of methane sulfonic acid.
We now have more novel chemistry available to overcome these challenges, resulting in more effective process improvements. This chemistry allows us to combine cleaning/deox/brightening steps and mitigate waste treatment problems.
Process improvements can and should be obtained by working on all aspects of an operation. In a metal finishing operation, the following offer the best improvements:
- New or innovative chemistry.
- Improved equipment.
- Better process control.
The focus here, however, will be on innovation in process chemistry. As mentioned earlier, acid-based chemistry is a key component in the finishing of copper alloys. We need acids, but not all acids are created equal. There are two general classes of acids that can used — mineral acids (inorganic) and organic acids. There are some classes of organic acids that can give us the best of both worlds, including sulfonic acids and methane sulfonic acid.
Based on the organic functional group, methane sulfonic acid can behave as a strong acid, similar to strong mineral acids, but without all the associated undesired properties and process problems. Another key feature of methane sulfonic acid is that it does not require chelate metals. This allows for spent cleaning solutions and rinses to be processed through standard waste treatment systems. Chelators can interfere with removal of metals from waste stream in a wastewater treatment system.

Brass parts before and after cleaning via oblique barrel. The new and improved process produced a brighter finish.
The surface preparation of nonferrous metals, such as copper and aluminum alloys, can be challenging and complex. The advancements in organic acid cleaners can help reduce the challenges associated with these alloys and also produce better quality parts in your process. This new organic acid chemistry technology, when formulated correctly, can contribute to significant process improvements such as reduced number of process steps, increased productively, quality improvements, and improvements in waste disposal and waste water treatment systems
About the Author
Mike Valenti is product manager – cleaners and nonferrous surface preparation at Hubbard-Hall. Visit hubbardhall.com.
RELATED CONTENT
-
Reducing Variation: The Only Way to Reduce Production Cost
Hidden variation in production is what increases costs in manufacturing. Here are four tips for reducing variability.
-
Deal Differently with Certainty, Risk and Uncertainty
Let's take a look at the differences between certainty, risk and uncertainty, examples of each, and how we make decisions when faced with these situations.
-
5 Grinding Considerations for Improving Surface Finish
Improving surface finish can be done by making adjustments to one or more of these points: operational parameters, wheel dressing, grit size, coolant delivery and machine condition.



