Is Cleaning with Solvent More Beneficial than Aqueous Cleaning?
To understand solvent-based and aqueous cleaning methods, Venesia Hurtubise, technical chemist, MicroCare Corp., has compiled the following details about both processes.
Edited by Lori Beckman
Aqueous cleaning has been used as a method for precision cleaning for a long time, but its efficiency and sustainability has been debated through the years compared with its solvent-based alternative. To understand both cleaning methods, Venesia Hurtubise, technical chemist, MicroCare Corp., has compiled the following details about both processes.
Aqueous Cleaning
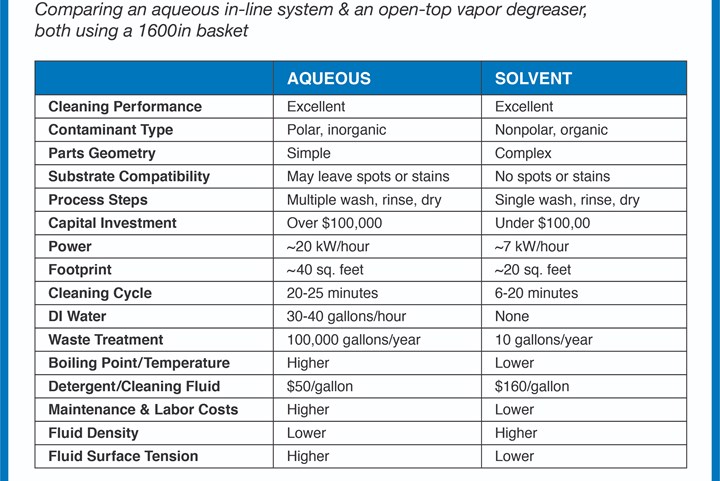
Aqueous cleaning, or water-based cleaning, is particularly useful when combining the cleaning process with other procedures such as depositing rust preventers and brightener coatings. However, because the power to clean with water alone is weak, water is treated with a variety of compounds, including detergents or surfactants, builders, emulsifiers, saponifers, sequestering agents and chelating agents to strengthen its cleaning ability.
Featured Content
This method can be effective at cleaning metal parts. However, aqueous systems produce a waste stream that requires treatment before discharge. Wastewater must be filtered, distilled, deionized and osmosis prepped for disposal. The cleaning agent added to the water can be toxic and nonbiodegradable, making it problematic to dispose of.
There is also the added environmental impact of using large amounts of water and power during the cleaning process. Aqueous systems require high temperatures to be effective, using more power. Because the machines are generally bigger than solvent-based systems, they consume more energy. Also, because water in some parts of the world is a precious non-renewable resource, aqueous cleaning scores low for environmental sustainability.
Aqueous cleaning is also harder to dry completely. This is especially true for parts with blind holes and small gaps. Water can leave unwanted stains behind or lead to corrosion forming.
Solvent Cleaning
An alternative to aqueous cleaning is solvent-based cleaning using a vapor degreaser. Modern vapor degreasers use updated environmentally progressive solvent to deliver consistent and safe cleaning results.
These fluids are engineered with the correct characteristics such as high densities, low surface tensions and low viscosities to wet every surface. Solvents can penetrate the most complex of shapes to completely dissolve contaminants and remove even the most stubborn particulate. They are also compatible with a range of metals and plastics and clean a variety of soils with ease. These solvents are chemically and thermally stable, meaning they do not turn acidic with use, and are nonflammable for workplace safety.
Parts come out of a vapor degreaser clean, dry, spot-free and cool enough for immediate further processing or packaging.
Contaminants: Match and Remove
To remove contamination successfully, it is important to match the cleaning fluid (aqueous or solvent) to the contaminant itself. To do so, it is critical to understand whether the contaminant is a particulate, or a polar (inorganic) or non-polar (organic) soil.
Particulate that will not dissolve in water or solvent-based cleaning fluids include metal shavings, dust, surfactants, stearates and polishing pastes. The particulate cannot be dissolved or solubilized and instead is displaced from the part and washed away. The cleaning fluid gets under the particulate, dissipates the static holding it to the part and removes it from the surface. This cleaning method is used in both aqueous and solvent-based processes.
Inorganic contamination includes salts, soaps, emulsion residue and graphite. It also encompasses oxidation such as rust and tarnish, heat scale, smuts, and carbonaceous and metallic compounds. These soils are soluble in water; therefore, aqueous cleaners are effective at removing the particulate because like dissolves like. Water-based detergents and surfactants emulsify and encapsulate contaminants so they can be washed away.
Organic soils are non-polar halogenated, oxygenated and hydrocarbon soils. Examples include machining and stamping oils, grease, corrosion protection agents and esters and baked-on resins. Organic contamination can be dissolved and removed with specialty solvent-based cleaning fluids.
It is critical to choose a cleaning fluid according to the containment and ensure that it is chemically similar to the contaminant itself.
When educated on the contaminant to be cleaned and pros and cons of both aqueous and solvent-based cleaning, knowing which cleaning fluid and therefore, process, to use for an application should be clear.
To learn more about vapor degreasing, watch this video from MicroCare Corp. here.
RELATED CONTENT
-
What Does 2021 Have in Store for the Parts Cleaning Industry?
With a new opportunity to do good things in your organizations this year, I hope you use the parts cleaning section and this year’s and last year’s Parts Cleaning Conference as tools to succeed.
-
Simple, Effective Parts Cleaning
After trying an array of parts cleaning methods over the years, this shop has implemented an environmentally friendly, relatively simple system to clean every part it produces.
-
The Importance of Drying Parts After Cleaning
Most cleaning processes consist of three steps, not two: wash, rinse and dry. That drying step is absolutely necessary for everything from product finishing to product performance to effective throughput to product quality.






