Post-Cleaning Corrosion Prevention
To keep corrosion away, understand that cleaning, passivation and corrosion protection are separate activities with distinct functions.
In an industry where competition among machine shops can be fierce, it’s critical to produce parts that are high quality and will stay that way for as long as possible. However, there are some obstacles that stand in the way of longevity and corrosion is one of them. Corrosion is the process by which metal surfaces are modified to form an oxidation product such as rust.
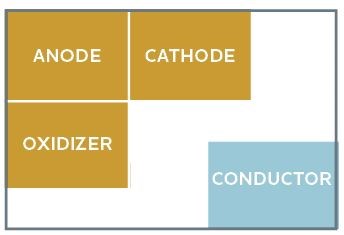
To understand how corrosion works, consider the Corrosion Square. The parts of the corrosion square (Fig. 1) consist of an anodic area that supplies electrons, a cathodic area that consumes electrons, a conducting path between the two and oxygen or an oxidizing chemical. To forestall corrosion, remove at least one component of the square (Fig. 2).
Surface preparation, notably critical cleaning, must be understood and managed properly. Otherwise, the corrosion square slips back together and corrosion happens. One key to success is understanding that cleaning, passivation and corrosion protection are separate activities with distinct functions. Cleaning is removing soil or unwanted matter, without damaging the surface. Passivation, typically surface treatment with an acid, is a modification that leaves the surface less susceptible to corrosion. The notion that passivation can replace cleaning is not true. If the soils are polar, such as salts, then passivation may be enough.
However, if metalworking fluids are used, usually non-polar, organic compounds are present. Acid treatment won’t remove most surface soils that accumulate during metal forming or fabrication. In fact, cleaning with water alone and then passivating is an invitation to corrosion. If the surface is not clean enough, passivation will not be consistent. There will likely be poor coating adhesion, leading to rejoining of the conductive portion to the corrosion square.
To effectively remove most soils, aqueous cleaning processes use basic (high pH) or sometimes near-neutral cleaning agents. Solvent cleaning is sometimes preferred, on the grounds that aqueous processes leave a part “too clean.” This is because while cleaning is an essential part of surface preparation, the soil, notably the metalworking fluid, acts as a non-conductive coating. The more effective the soil removal, the higher the potential for corrosion.
It is becoming more difficult to clean a product, in part because metalworking fluids have evolved and leave more residue. Manufacturers used to use “vanishing oils.” These oils left some residue on the part that protected the product from corrosion. The downside was that the oils vanished into the air, resulting in pollution. Given environmental mandates, metalworking fluids were reformulated to be less evaporative, which means they leave more complex residue on the part. This residue can interfere with subsequent assembly, coating and finishing operations and must be removed using aggressive cleaning, resulting in a surface that is susceptible to corrosion.
One solution is to clean and then forestall corrosion using a corrosion inhibitor to reduce conduction. However, any corrosion inhibitor is adding a contaminant to the surface. The right corrosion inhibitor depends on the customer. In an era of increased cleaning costs and diverse customer requirements, those who fabricate, stamp or machine metal parts may, out of necessity, avoid making a decision about the correct cleaning process or the optimal corrosion inhibitor. The machinist may not do any cleaning and/or may supply the product packed in oil. In this case, cleaning processes may require higher temperatures, stronger physical forces, and longer cleaning and rinsing times than normal. More aggressive cleaning can lead to corrosion susceptibility.
For complex supply chains, the customizing corrosion protection for a range of customers may require finesse. However, where there is a captive or dedicated machine shop, there is a simple, radical solution: communicate and coordinate!
Related Content
3 Common Filtration Questions Answered
Learn about the variety of filters for removing particulates from a cleaning fluid, how to determine cleaning fluid life and more.
Read MoreOvercoming 3 Common Challenges With Automated Particle Counting
Facing difficulties while performing particle analysis is normal but should not be discouraging. Here are some ways to handle the most prevalent issues that can arise.
Read MoreMiraclean Tanks Enable Highly Configurable Cleaning System
PMTS 2025: Miraclean’s cleaning systems consist of individual ultrasonic or static tanks in a standard size that can be organized within a stainless-steel frame.
Read MoreKyzen Solvents Provide Safe Parts Cleaning
The SLV901 and SLV803 solvents are formulated to maintain cleaning efficacy while providing a safe, environmentally friendly alternative to processes that use PFAS and HFCs.
Read MoreRead Next
How To (Better) Make a Micrometer
How does an inspection equipment manufacturer organize its factory floor? Join us as we explore the continuous improvement strategies and culture shifts The L.S. Starrett Co. is implementing across the over 500,000 square feet of its Athol, Massachusetts, headquarters.
Read MoreFinding the Right Tools for a Turning Shop
Xcelicut is a startup shop that has grown thanks to the right machines, cutting tools, grants and other resources.
Read More